Co-crystallisation is a fascinating process wherein two or more different molecules are combined to create a new crystalline structure. This technique has gained enormous importance in the pharmaceutical industry due to its potential for enhancing the physical and chemical properties of drugs, including solubility, stability, and bioavailability. Here, we’ll provide a brief overview of co-crystallisation, exploring its fundamental principles, applications in drug development, and the products that support this process.
The Basics of Co-Crystallisation
Co-crystallisation is all about developing new structures called co-crystals. The properties of these novel structures can be influenced by numerous factors, including intermolecular interactions, hydrogen bonding, and π-π stacking.1 With this in mind, it’s crucial that specific reaction parameters can be tightly controlled to ensure consistency in results. For example: morphology, polymorphism, and size distribution are factors that underlie crystal quality – perhaps the most important property of all. Producers can control crystal quality through temperature cycling, which involves cycles of heating and cooling crystals to maintain their properties.
Understanding the various factors that affect co-crystallisation is essential for successful implementation. The choice of co-formers, for example, is essential. Co-formers are selected based on their ability to form non-covalent interactions with the active pharmaceutical ingredient (API). Screening techniques, including solid-based and solution-based methods, also help to identify potential co-crystals. Additionally, characterisation techniques like X-ray diffraction and spectroscopy are then used to determine the crystal structures and properties of the co-crystals.
Types of Co-Crystals
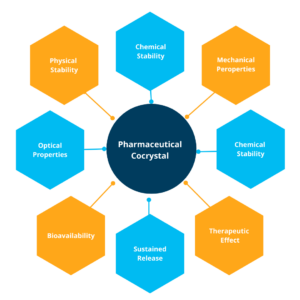
Co-crystals can take various forms depending on the components involved in the process. Pharmaceutical co-crystals, in particular, have garnered significant attention in drug discovery and development. These co-crystals consist of an API and a pharmaceutically acceptable co-crystal former. The resulting nonionic supramolecular complexes offer unique properties that can address challenges related to drug solubility, stability, and bioavailability.
Applications of Co-Crystallisation
Co-crystallisation is relevant to more than just the pharmaceutical industry; It factors into textiles, paper, chemical processing, and more. Their significance in the pharmaceutical field cannot be overstated though. Co-crystals have emerged as potential alternatives to many traditional solid forms of pharmaceuticals. By modifying the molecular interactions and composition of pharmaceutical materials, co-crystals can enhance the physicochemical properties of APIs.2 They offer opportunities to improve solubility, physical stability, and mechanical properties without changing the chemical composition, ensuring better efficacy and patient compliance.
Products to Support Co-Crystallisation
Developing co-crystals is an advanced technique requiring specialised instrumentation. The Integrity 10 Reaction Station is an innovative tool that enables researchers to run ten separate reactions simultaneously, with precise control over temperature and stirring rates for each individual reaction. This system accelerates investigative chemistry by offering flexibility in designing temperature profiles for individual experiments. It proves valuable in high-throughput chemistry, as it can be used for the following (amongst others):
- Catalyst screening
- Kinetic studies
- Polymorph screening
- Reaction optimisation
- Solubility testing with turbidity measurements
- Stability studies
The Integrity 10 reaction station boasts user-friendly features, including a touchscreen menu and pre-programmed profiles for routine measurements. It can also be customised and upgraded according to specific requirements, offering an expansive range of accessories and optional features like full automation or high-pressure screening. Despite those extensive functionalities and high-throughput capabilities, the Integrity 10 offers world-class energy efficiency. Each cell is pre-programmed to use an exact energy input and can switch off automatically, reducing energy consumption by up to 90% compared to traditional techniques.

With advancements in equipment like the Integrity 10 reaction station, scientists have access to innovative tools that accelerate research and provide valuable insights into the co-crystallisation process. By harnessing the potential of co-crystals, the pharmaceutical industry can continue to advance drug discovery and improve patient outcomes. Excited to learn more? Contact the Asynt team today.