Why is flow photochemistry more sustainable than other methods?
Chemical reactions are generally activated by heat or chemical reagents. Recent developments in laboratory equipment have enabled less common methods to become more accessible: methods such as electrochemistry and photochemistry. Tools such as a flow photoreactor uses light as a reagent, allowing for a cleaner and therefore greener reagentless (also known as traceless) chemistry.
These improvements in technology have allowed for a significant increase in the use of photochemistry and the number of publications in this area. This increase has also been encouraged with the potential in developing of novel synthetic routes (reducing the number of reaction steps) and a growing range of novel photoredox catalysts. Reducing the number of steps saves material resources, time and energy, and reduces waste. Further refinements in reactor design and improved light source technology will hopefully advance these sustainable aspects even further in future, and this is something that our own R&D department is working on.
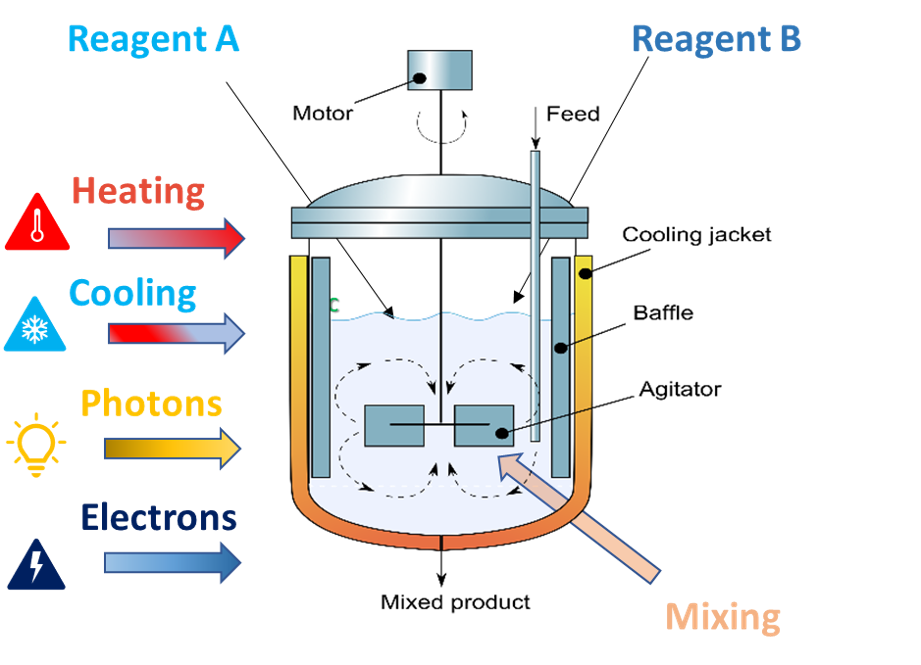
A diagram of a CSTR
Light sources for photochemistry
Traditionally, mercury-vapour lamps have been used for photochemistry. There are some drawbacks of using these as a light source: they emit a wider spectrum of light with multiple wavelengths, are energy inefficient and require increased safety precautions to protect the user from harmful irradiation. As a result, LEDs have become the go-to option for photochemistry, especially as the technology has so drastically improved in recent years.
LEDs have much narrower peaks at discrete wavelengths, allowing for better selectivity and optimisation of reactions. They are also safer (although safety precautions are still paramount when using very intense or ultraviolet light) and more efficient. All of our photoreactors feature LEDs – either individually or arrayed, with a wide selection of wavelengths available.
A further significant benefit to highlight is that flow systems tend to have a smaller volume of reactant active at any given time, thus reducing potential hazards from spills or exothermic reactions.
Our Illumin8 photoreactor with additional LED module
Flow photochemistry reactors
Irradiation is a crucial factor in photochemistry but effects rapidly reduce over any given distance. Batch photochemistry reactors solve this by mixing their reactants, placing the light source as close as possible to the reaction, or directly channelling the photons into the reaction medium in the case of our LightSyn Lighthouse reactor.
This is where flow reactors provide a key advantage. The narrow diameter of capillaries and tubes allows for a much higher level of light penetration, ensuring rapid and even irradiation of your reactants. By coiling the tubing around the light source, such as in the case of our Borealis, you can also run your reaction for an increased residence time while it’s being irradiated.
Flow photochemistry also allows for more controlled and homogenous reactions, enabled by precise stoichiometric mixing of reactants before they are pumped through the photo reactor.
Another method for combining flow and photochemistry is to irradiate continuous stirred tank reactors (CSTRs). CSTRs allow for a degree of multiphasic reaction conditions including solid components. In the case of our flow photoreactor, fReactor PhotoFLOW, you can position up to 5 LED modules (of your preferred wavelength) with one on each CSTR. These safely shine only through the CSTR’s windows, directly into the reaction.
Cutaway image of our Borealis flow photoreactor showing the internal LED array
Conclusion
Flow photoreactors represent an excellent opportunity to enhance photochemical processes, reducing waste and increasing throughput all with a safer and more precise flow process. Continued advances in technology and synthetic routes open the future to exciting possibilities for new research.
Ready to “glow with the flow”? Speak with us via live chat now or email [email protected]. We look forward to hearing from you!